General Questions and Answers Concerning OECD Principles of Good Laboratory Practice (GLP) and Mutual Acceptance of Data (MA
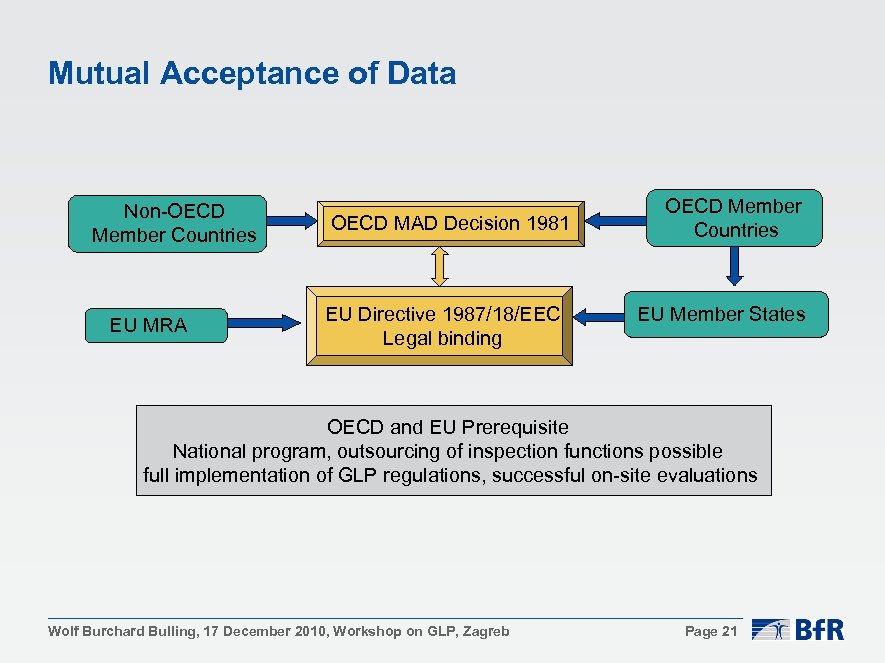
Official Journal of the European Communities 9. 10. 1999 L 263/10 AGREEMENT on mutual recognition of OECD principles of good lab